Why Does Bcl3 Have A Trigonal Planar Shape
Why does BCl3 have trigonal planar shape. BCl3 has three B-Cl polar bond.

Hybridization Of Bcl3 Hybridization Of Boron In Bcl3
The nonbonding pair of electrons pushes away from the bonding pairs producing a trigonal pyramidal shape.

Why does bcl3 have a trigonal planar shape. It does not dimerize although NMR studies of mixtures of boron trihalides shows the presence of mixed halides. The reason is very simple and is stated as follows-. The geometry of AlCl3 is trigonal planar.
The molecule has three bond pair electrons which arrange themselves in a trigonal planar way to avoid the repulsion. However Boron only has 3 electrons to bond. Here NH3 Nitrogen has 5.
BCl 3 is a trigonal planar molecule like the other boron trihalides and has a bond length of 175pm. There are 4 electron pairs but from the molecule we can see there are 3 bonding pairs so there must be 1 lone pair. This problem has been solved.
Thus B-Cl3 does not have a dipole moment. With all 120 degrees apart with a pull that each exerts is balanced out. The BCl3 molecule is considered to be non-polar because the charge distribution across the molecule is uniform as the shape of the molecule is symmetric ie.
No3- have 4 bonded pairs and 8 lone pairs. BCl3 has 3 B-Cl single bonds and no lone pair around B hence 6 valence electrons around B. Trigonal Planar Molecular Geometry.
Chapter 23 Problem 27E is solved. This video reviews how to draw Lewis structures for molecules. Its a 4 marker asking explain why a BF3 molecule has the shape you have drawn Please help.
Therefore there exists no polarization of charges across the BCl3 molecule. If the central atom with no lone pair is bonded to three other atoms the molecule will have a trigonal planar shape. Boron trichloride BCl3 is characterized by trigonal planar molecular shape.
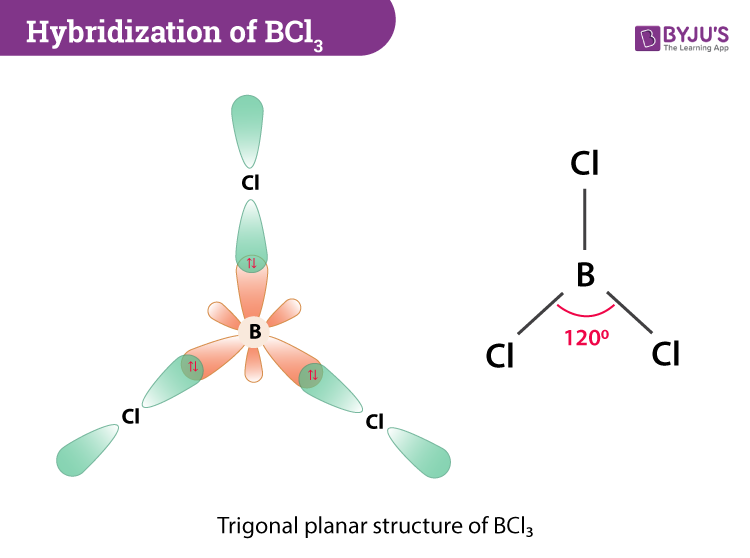
This molecule is made up of 3 equally spaced sp 2 hybrid orbitals arranged at 120 o angles. Probably marks for saying. The bond angle is 120 o.
It is composed of 1 boron and 3 chlorine atomThe bond angles are 120. - lone pairs repel more strongly than bonded pairs. - repel each other equally at 120 degrees.
In BCl3 Boron has a total of 3 valence electrons. When you will apply this formula the hybridisation will come out to be sp3d with condition of 2 lone pair and 3 bond pair so as to attain maximum stability and minimum repulsion the lone pairs are placed at the equitorial position and hence the shape of compound will be T shape Well more precisely itll be Bent T shaped reason again to minimize the repulsion. If we look at the structure BCl 3 molecular geometry is trigonal planar.
- no lone pairs. Bf3 has planar shape while NH3 has pyramidal because B has 3 electrons in the outermost shell while N has 5 elctrons in its outermost shell. Read More About Hybridization of Other Chemical Compounds.
The best configuration for bonding with chlorides that maximizes that distance between these two atoms is trigonal planar. The bond dipoles of three B-Cl bonds give a net sum of zero because the resultant of any two is equal and opposite to the third. As per VSEPR notation this molecule takes AX3 notation.
Molecules with the trigonal planar shape are triangular and in one plane or flat surface. Nitrogen is pentavalent and in NCl 3 Nitrogen has a lone pair where as Boron is trivalent and in BCl 3 no lone pairs are present so BCl 3 has trigonal planar shape and NH 3 has trigonal pyramidal shape. NCl3 therefore has 3 bonds and 1 lone pair so 4 things to show in the bond diagram.
- 3 bonded pairs. BCl3 has 3 B-Cl single bonds and no lone pair around B hence 6 valence electrons around B. Hence in NH3 1 lone pair of electron is present and due to repulsion between lone pair and bond pair its shape become pyramidal.
Making it trigonal pyramidal. The shape of the orbitals is planar triangular. This means the shape is trigonal pyramidal.
A degree of π-bonding has been proposed to explain the short B Cl distance although there is some debate as to its extent. - the molecules are all aligned in one plane - essentially the molecule is flat. Since there is an atom at the end of each orbital the shape of the molecule is also planar triangular.
The repulsion between these will be at a minimum when. BCl3 have trigonal planar symmetrical geometry. The three atoms bound to the central atom represent the vertices of a triangle.
You can see the lone pair nonbonding of electrons directly above the nitrogen. Why does no3- have a molecular geometry shape of trigonal planar. Isnt it true that In order to be a trigonal planar there should be 4 bonded pairs and 0 lone pairs.
The geometry of BCl3 is trigonal planarAs per VSEPR notation this molecule takes AX3 notation. An AX 3 molecule such as BF 3 has three regions of electron density extending out from the central atom. The central atom also has a symmetric charge around it and the molecule is non-polar.
What Is The Structure Of Bcl3 Quora
Bcl3 Lewis Structure Molecular Geometry Hybridization And Shape

Using Vsepr To Determine Molecular Shape Bcl3 Intermolecular Forces Meristem Youtube
Hybridization Of Bcl3 Hybridization Of Boron In Bcl3

Is Bcl3 Polar Or Nonpolar All About Bcl3 Polarity Shape
What Are The Hybridisation And Shape Of Bcl3 Quora
What Is Hybridization How Does It Explain The Formation Of Bcl3 And Sf6 Molecules Quora
What Is The Structure Of Bcl3 Quora

Why Is Bcl3 Trigonal Planar In Shape Whereas Anhydrous Alcl3 Is Tetrahedral In Shape Chemistry Chemical Bonding And Molecular Structure 12837253 Meritnation Com
0 Response to "Why Does Bcl3 Have A Trigonal Planar Shape"
Post a Comment