What Is The Electron Geometry Of Clf3
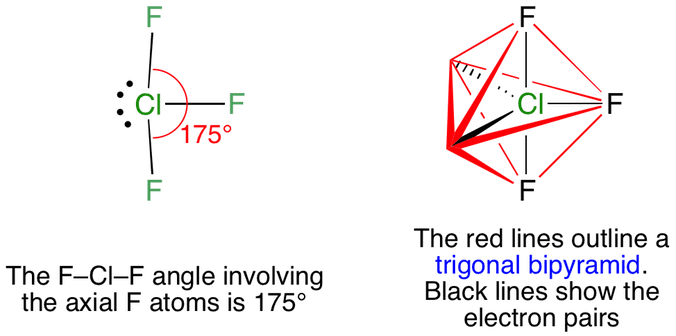
These are arranged in a trigonal bipyramidal shape with a 175 F axial-Cl-F axial bond angle. The molecular geometry of ClF3 is T-shaped and electron geometry is Trigonal bipyramidal.
Clf3 Chlorine Trifluoride Molecular Geometry Bond Angles Electron Geometry Youtube
What is the electronic geometry of ClF_3 and BI_3.

What is the electron geometry of clf3. The structure of ClF3 is given below. This provides the lowest energy regulation of the electron pairs in the molecule. The electron geometry of chlorine trifluoride is trigonal bipyramidal with a 175 F-Cl-F bond angle.
Enter the electronic geometry of the molecule. What Is The Electronic Geometry Of ClF_3 And BI_3. Click and drag the molecle to rotate it.
For trigonal bipyramidal electron pair geometry lone electron pairs should occupy the equatorial positions not the axial positions to attain minimum electronic repulsion. The molecule of chlorine trifluoride is as. Chlorine trifluoride appears as a colorless gas or green liquid with a pungent odor.
So the electron pair geometry of ClF3 will be trigonal bipyramidal. Click and drag the molecle to rotate it. See full answer below.
Then draw the 3D molecular structure using VSEPR rules. We review their content and use your feedback to keep the quality high. The electronic geometry of the molecule Best Answer.
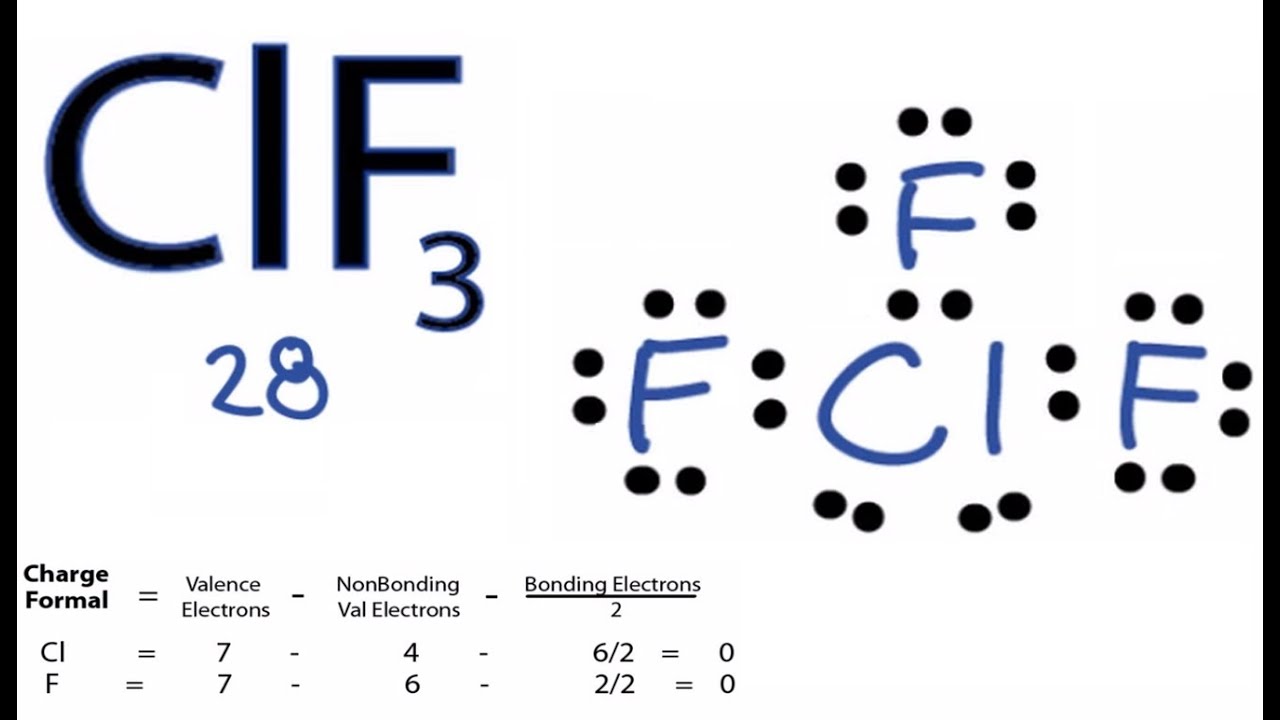
The central atom chlorine forms a single bond with three fluorine atoms with additional. First draw the Lewis dot structure. ClF3 - Chlorine Trifluoride.
So out of 5 positions two F atoms occupy two axial positions and rest F. Both of them belong to group 7 of the periodic table and have a valency of 7. It reacts with water to form chlorine and hydrofluoric acid with release of heat.
Previous question Next question. Our tutors rated the difficulty of Determine the molecular geometry of ClF3. The geometry is square pyramidal and is due to 6 electrons pairs around the central chlorine atom one of which is nonbonding.
100 35 ratings Transcribed image text. Chlorine trifluoride has 5 regions of electron density around the central chlorine atom 3 bonds and 2 lone pairs. Total number of valence electrons in ClF3 7 73 28.
Basic trigonal bipyramid structure T-shaped structure CLF3 indicating the orbit of a single pair. The actual structure of clf3 with slightly bent angles. There is also an asymmetric charge distribution around the central atom.
Chlorine trifluoride has an electron geometry of T-shaped. The equatorial position at an angle of 120 to each other. ClF3 molecular geometry is said to be a T-shaped.
What is the difficulty of this problem. It is a triangular pyramid in which only one corner of the triangle is occupied by F atom and the other two are occupied by two lone pairs of electrons. ClF3 has one chlorine atom and three fluorine atoms.
ClF3 is polar in nature. The total valence electron present in the chlorine trifluoride is 28. Or if you need more Electron Geometry practice you can also practice Electron Geometry practice problems.
What is the electron geometry of ClF3. According to the lewis structure of ClF3 2 lone pairs and 3 bonded pairs present in this molecule. ClF3 - Chlorine Trifluoride.
A Cl-F bond is a dipole since F is more electronegative. The Electronic Geometry Of The Molecule This problem has been solved. What is the molecular geometry for ClF3.
Chlorine has an atomic number of 17 and Fluorine has an atomic number of 9. Chlorine Trifluoride ClF3 Molecular Geometry Polarity. The molecular geometry of ClF3 is T-shaped with asymmetric charge distribution around the central atom.
In the hybridization of chlorine in ClF3 we get 28 electrons where 14 electron pairs are distributed around the central least electronegative chlorine atom then we get ClF3Two lone pairs are associated with the central chlorine atom and therefore form a trigonal bipyramidal electronic geometry. Contact with organic materials may result in spontaneous ignition. First draw the Lewis dot structure.
The two apexes are occupied by one F atom each. Sp3d Then draw the 3D molecular structure using VSEPR rules. It acquires such shape because of the presence of two lone pairs which take up equatorial positions and there are greater repulsions.
What is the electronic geometry of H2O. Experts are tested by Chegg as specialists in their subject area. It appears to be asymmetrical and is polar.
What Is The Molecular Geometry Of Cif3 Ci Not Cl Quora

What Is The Molecular Geometry Of Clf3 Quora

Clf3 Chlorine Trifluoride Molecular Geometry Bond Angles Electron Geometry Youtube

Hybridization Of Clf3 Hybridization Of Cl In Chlorine Trifluoride

Hybridization Of Clf3 Hybridization Of Cl In Chlorine Trifluoride

Clf3 Chlorine Trifluoride Molecular Geometry Bond Angles Electron Geometry Youtube
Vsepr Clf3 Chlorine Trifluoride
What Is The Electronic Geometry Of Clf3

What Is Molecular Geometry Of Ab5 And Ab6 Type Compounds
0 Response to "What Is The Electron Geometry Of Clf3"
Post a Comment