How To Make Vsepr Structures
VSEPR theory is a set of rules f. Two points in space will make a line Each atom likes having 8 electrons around it since all noble gases have 8 electrons.

Vsepr Theory Basic Introduction Youtube
143 Apply the VSEPR model to predict basic shape The VSEPR model can be applied systematically by following a few simple steps.

How to make vsepr structures. In this video were going to apply VSEPR theory to six electron clouds so if our goal is to find the shape of the sulfur hexafluoride molecule once again we start with our dot structure so sulfur is in group 6 on the periodic table so six valence electrons fluorine is in group seven so seven valence electrons but I have six of them so seven times six gives me 42 and 42 plus six gives me 48 valence electrons that we need to show in our dot structure. Electron pairs gives base shape Octahedral VSEPR base shape for 6 e-pairs Refcode. Identify the central atom 2.
It contains examples and practice problems of drawing. How to Determine a VSEPR Structure 1 Draw the Lewis Structure. The valence shell electron pair repulsion VSEPR theory gives us a qualitative explanation for the covalent molecules particular geometries.
Chapter 5 Lesson 11. Divide the total of these by 2 to find the total number of electron pairs. Each group around the central atom is designated as a bonding pair BP or lone nonbonding pair LP.
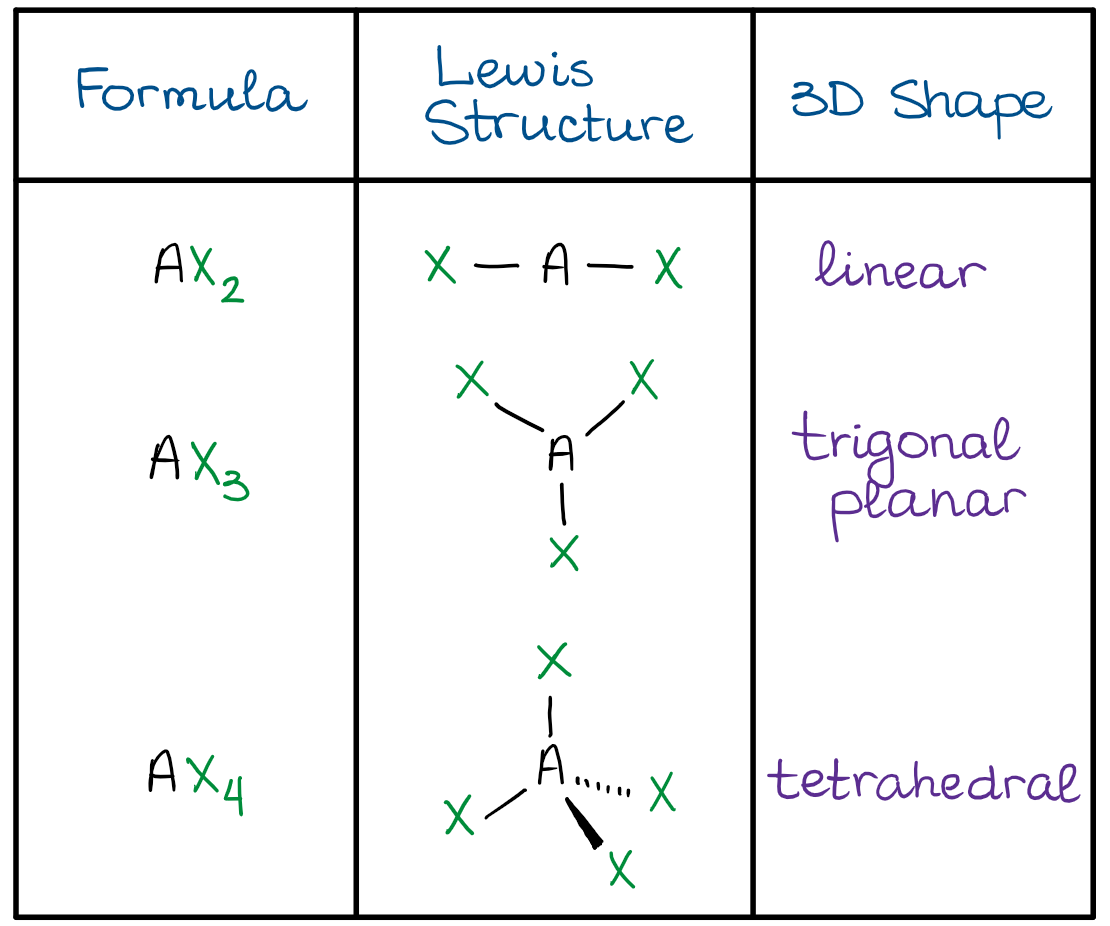
Add one electron for each bonding atom 4. In the VSEPR model the molecule or polyatomic ion is given an AX m E n designation where A is the central atom X is a bonded atom E is a nonbonding valence electron group usually a lone pair of electrons and m and n are integers. How to draw lewis structures and vsepr.
This table is more complete than the table found in your. The general concept is that the pairs of electrons repel each other and try to locate themselves as far as possible from each other about a given nucleus. Add or subtract electrons for charge see Top Tip 5.
Using the VSEPR Model. First draw the Lewis structure of the molecule and identify the central atom. Molecular Structures and VSEPR Theory.
3 Determine the basic arrangement of all electron density regions bonds. The bond is drawn as a line or dash. Steps to draw a lewis structure step 1.
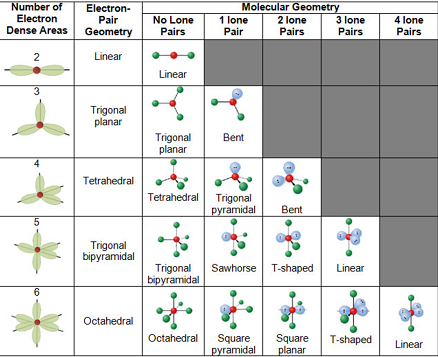
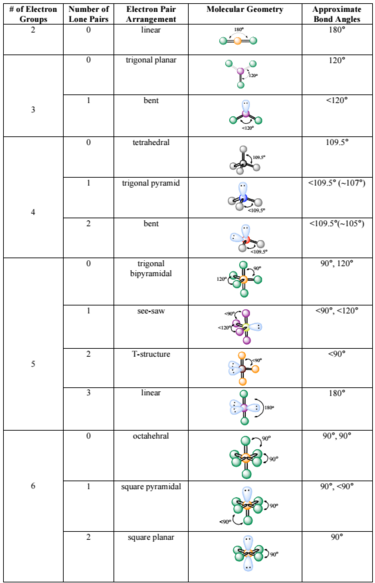
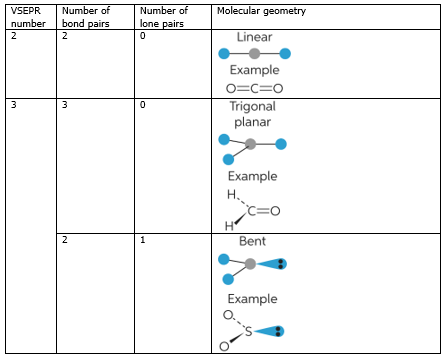
Molecule methane ethene ethyne 4 aminobenzoic acid Lewis Structure Look it up VSEPR. The line or dash equals 2 electrons For the shape the atoms are in a linear shape. Based on correct Lewis Dot structures use the table described below to determine its number of VSEPR electron domains predicted shape and polarity.
As an example we will consider the structure of hexafluorophosphate PF6-. You should print a figure using VSEPR to predict molecule geometry shape polarity and electron domain arrangments for molecules having Three to Six Electron Domains. Choose a central atom.
2 Count the bonds and lone pairs about the central atom. Count its valence electrons 3. The shapes of thesemolecules can be predicted from their Lewis structures howeverwith a model developed about 30 years ago known as the valence-shellelectron-pair repulsion VSEPR theory.
The VSEPR theory assumes that each atom in a molecule willachieve a geometry that minimizes the. Find the number of electrons needed to make the atoms happy. Lewis dot structures can be drawn to show the valence electrons that surround an atom itself.
B trigonal planar trigonal planar. This chemistry video tutorial provides a basic introduction into VSEPR theory and molecular structure. 2 electrons will make a bond.
In this lesson youll learn about the VSEPR theory and how it can be used to explain molecule shapes. Solution continued As this example illustrates when a molecule exhibits resonance any one of the resonance structures can be. A step-by-step explanation of how to draw the IO3- Lewis Dot Structure Iodate IonFor the IO3- structure use the periodic table to find the total number of.
Hence for two pairs of electrons on a nucleus the two pairs would locate themselves exactly opposite each other forming a. Note that multiple bonds are counted as one region of electron density. Then learn how to predict the shape of a molecule by applying.
The Valence Shell Electron Pair Repulsion Theory VSEPR helps us to understand the 3D structure of molecules. Predict the electron-domain geometry and the molecular geometry for a SeCl. For PF6-the central atom is a phosphorus.
BO½842 YES a BO of 2 predicts the double bond from VSEPR and VB. Join the one Na electron with one electron from Cl to make a bond. Diamagnetic Paramagnetic Fill in the chart below.
There is no direct relationship between the formula of acompound and the shape of its molecules.

Molecular Geometry Boundless Chemistry
Greenhouse Gases Vsepr Theory Courtney S Chemistry
Vsepr Theory Organic Chemistry Tutor

Valence Shell Electron Pair Repulsion Theory Vsepr
Vsepr Theory And Molecular Geometry Ch4 Nh3 H2o Youtube
Learn About Vsepr Model Chegg Com
Greenhouse Gases Vsepr Theory Courtney S Chemistry
Vsepr Theory Postulates Limitations Predicting Shapes
0 Response to "How To Make Vsepr Structures"
Post a Comment