The Shape (molecular Geometry) Of Bcl3 Is
If we look at the structure BCl 3 molecular. This is because the molecular arrangement of the chlorine atom is part of a complete triangular shape.
What Is The Molecular Geometry Of Bcl3 Quora
Chemistry questions and answers.
The shape (molecular geometry) of bcl3 is. Use lewis structure guidelines to draw the lewis structure of BCl 3. What is the molecular geometry of BCl3. The bond angle is 120o.
This video tutorial will explain how to draw the Lewis dot structure and molecular geometry for boron trichloride BCl3. Molecular geometry is trigonal planar. Thionyl tetrafluoride is an inorganic compound gas with the formula SOF4.
Chemistry questions and answers. The boron is located in the center which has three valence electrons and balances out the three chlorine. 18919 views around the world.
What is the structure of BCl3. In its most stable state Boron forms three covalent bonds with the surrounding Chlorine atoms making for three bonded pairs in the centre. In the Lewis structure for BCl3 the central atom Boron will only have six valence electrons.
A Lewis structure is first structure and has two extra lone pairs on the central atom. Note that Boron can have a full outershell with only six valence electrons. 1 Answer Humaam H.
So the shape of BCl 3 molecule is trigonal planar. Chemistry Molecular Orbital Theory Molecular Geometry. AX 3 has trigonal planar shape.
Jul 12 2014 Answer link. The shape of the molecule is a distorted. A trigonal pyramidal polar B trigonal pyramidal nonpolar C trigonal planar polar D trigonal planar nonpolar E trigonal bipyramidal polar.
BCl 3 has a trigonal planar structure with bond angles of. If we look at the structure BC l3. 1 The Molecular Geometry Of The BCl3 Molecule Is And This Molecule Is A Trigonal Pyramidal.
1 The Molecular Geometry Of The BCl3 Molecule Is. In the Lewis structure for BCl3 the central atom Boron will only have six valence electrons. This is free chemistry help for you.
See all questions in Molecular Geometry Impact of this question. Determine the electron geometry eg and molecular geometry mg of BCl3 eg trigonal bipyramidal mg tetrahedral eg trigonal planar mg trigonal planar eg trigonal planar mg tetrahedral eg tetrahedral mg trigonal pyramidal. The molecular geometry of BCl3 is trigonal pyramidal.
The angle of connection is 120 degreesA symmetrical load surrounds the center atom and the molecule is non-polar. C Molecular shape is T shaped. -trigonal pyramidal polar -trigonal planar polar -trigonal bipyramidal polar -trigonal pyramidal nonpolar -trigonal planar nonpolar.
BCl3 Molecular Geometry According to VSEPR theory the molecular geometry of boron trichloride is trigonal planar with a bond angle of 120 degrees. CO2 has a linear molecular geometry with the bond angles of 180 degrees and symmetric distribution of electrons. Draw its VSEPR and Lewis structure.
What is the structure of BCl3. The molecular geometry of the BCl3 molecule is _____ and this molecule is _____. BCl3 is a trigonal planar molecule like the other boron trihalides and has a bond length of 175pm.
Note that Boron can have a full outershell with only six valence electrons. BCl 3 consists of one Boron atom and three Chlorine atoms. 1 The molecular geometry of the BCl3 molecule is and this molecule is - A trigonal pyramidal polar B trigonal pyramidal nonpolar C trigonal planar polar D trigonal planar nonpolar E trigonal bipyramidal polar 2 Which one of the following is often easily separated into its components by simple techniques such as filtering or decanting.
A quick explanation of the molecular geometry of OH- the Hydroxide io including a description of the OH- bond anglesLooking at the OH- Lewis structure we. The molecular geometry of the BCl3 molecule is _____ and this molecule is _____. B VSEPR 3 bp 2 lp 5 shape is trigonal bipyramidal.
BCl3 molecular geometry is trigonal planar when we look at the structure. BCl 3 has an sp 2 hybridization state. Valence Shell Electron Pair Repulsion Theory VSEPR is used to determine the shape and bond angle of a molecule.
Use VSEPR table to find the shape. It is also known as sulfur tetrafluoride oxide. BCl3 is a trigonal planar molecule like the other boron trihalides and has a bond length of 175pm.
5 rows BCl 3 Molecular Geometry And Bond Angles.

Hybridization Of Bcl3 Hybridization Of Boron In Bcl3

Bcl3 Lewis Structure Molecular Geometry And Hybridization Techiescientist
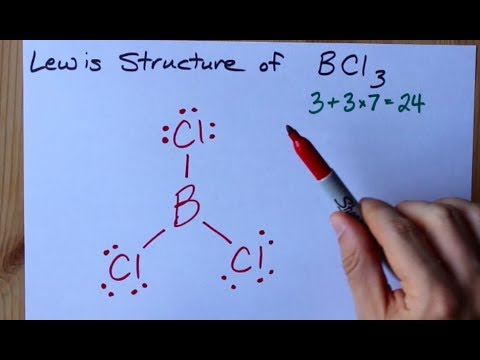
How To Draw The Lewis Structure Of Bcl3 Boron Trichloride Youtube

Bcl3 Lewis Structure Molecular Geometry And Hybridization Techiescientist
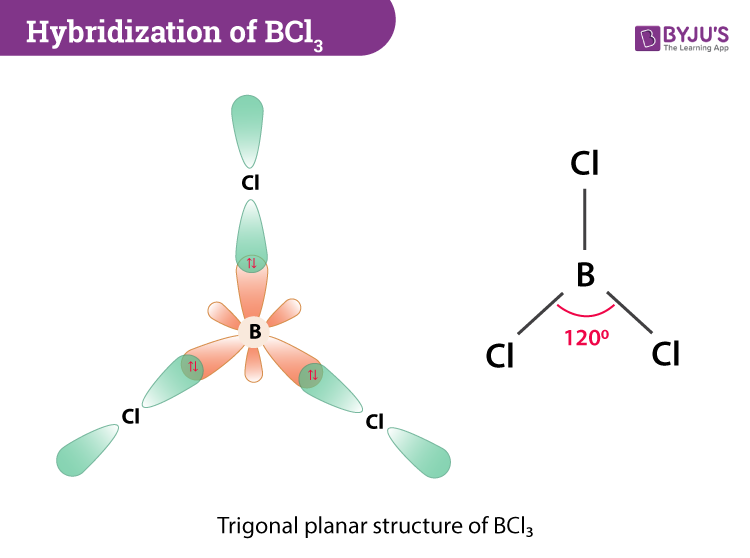
Hybridization Of Bcl3 Hybridization Of Boron In Bcl3
Bcl3 Lewis Structure Molecular Geometry Hybridization And Shape

Bcl3 Lewis Structure Molecular Geometry And Hybridization Techiescientist

Bcl3 Boron Trichloride Molecular Geometry Bond Angles And Electron Geometry Youtube

Using Vsepr To Determine Molecular Shape Bcl3 Intermolecular Forces Meristem Youtube
0 Response to "The Shape (molecular Geometry) Of Bcl3 Is"
Post a Comment